PathPresenter Achieves CE Mark for IVDR Compliance with Digital Pathology Clinical Viewer

Regulatory milestone clears path for expansion in Europe
New Jersey — October 23, 2025 — PathPresenter (www.pathpresenter.com), a leading Image Management System and workflow platform for digital pathology, is proud to announce that the PathPresenter Clinical Viewer has met the requirements of the EU In Vitro Diagnostic Regulation (IVDR 2017/746) and can now bear the CE mark. This certification clears it for clinical use in primary diagnosis in Europe.
The Clinical Viewer is a key part of PathPresenter’s vendor-agnostic digital pathology image management system (IMS), a comprehensive platform that includes not just slide viewing, but also workflow management, case tracking, image storage and archiving, integration with laboratory information systems (LIS), multi-user access, collaboration tools, and integrated remote second opinion capability.
“Successfully meeting In Vitro Diagnostic Regulation (IVDR) requirements not only affirms the rigor of our clinical viewer but also underscores PathPresenter’s readiness to operate at a global scale,” said Brian Matcheski, Head of Regulatory at PathPresenter. “Coupled with our recent FDA 510(k) clearance, this milestone reflects the growing trust in our clinical solutions and paves the way for meaningful expansion into the European and international markets.”
PathPresenter’s powerful platform, designed to facilitate viewing, sharing, and collaborating on whole slide images, has been purpose-built to bridge gaps and integrate previously siloed systems. It offers a robust, secure, tech-forward platform that takes maximum advantage of whole slide imaging and digital workflows to serve the ultimate goal of a clinician’s work: to provide the best clinical care.
To learn more about the PathPresenter platform for clinical use, visit https://www.pathpresenter.com/clinical-care/.
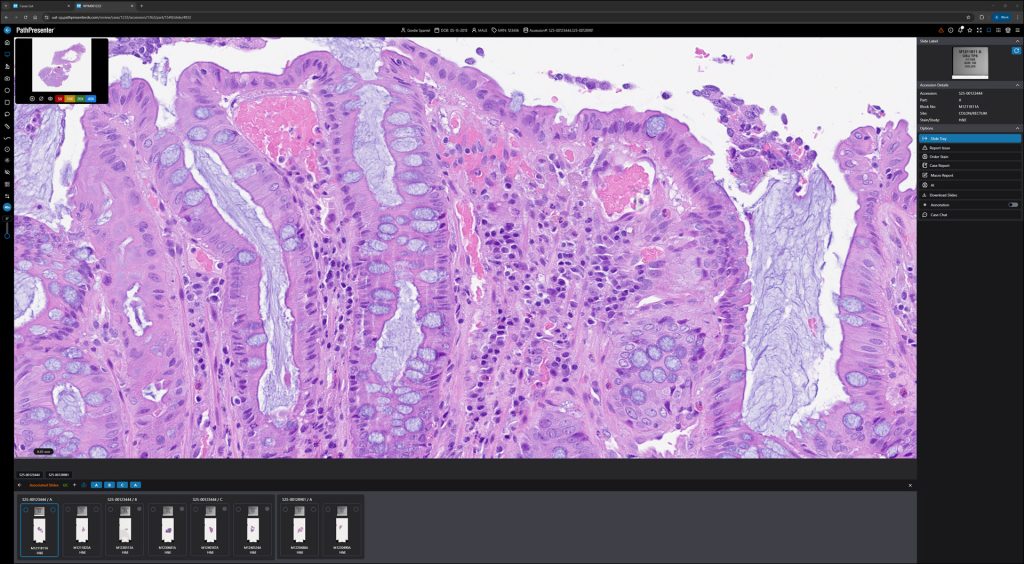
PathPresenter’s Clinical Viewer now carries both FDA 510(k) clearance and CE-IVDR certification.
About PathPresenter
PathPresenter is an enterprise image management and workflow platform for digital pathology. We are on a mission to democratize access to the world’s pathology knowledge by connecting pathologists to the vast expertise of their colleagues globally and providing a practical platform to access and use best-in-class AI models. Founded by dermatopathologist and digital pathology pioneer Dr. Rajendra Singh, PathPresenter’s clinical viewer has been granted FDA 510(k) clearance for primary diagnosis when paired with qualified digital scanners and its secure, scalable, vendor-agnostic enterprise pathology workflow software has been adopted by tier one medical institutions for clinical care, education, and research. The company has built a thriving community of tens of thousands of users around the world to easily view and share digital pathology images and knowledge. Learn more at www.pathpresenter.com.
Media Contacts
Neil Humphrey, VP of Marketing
neil@pathpresenter.com
+1 519 498 3402
More Posts
- Digital Pathology Basics: What is Digital Pathology
- Behind the Scenes of a Successful FDA 510(k): PathPresenter’s Clinical Viewer Journey
- The Digital Pathology Illusion: Why Are We Building Storage Instead of Solutions?
- Success Story: Accelerating Clinical Trials for Oncology with Imagenomix Predict and PathPresenter ConsultConnect
- Why Seamless IMS-LIS Integration Is Essential for Digital Pathology
